World
New COVID-19 Treatment Ratutrelvir Shows Promise Against Paxlovid

A mid-stage clinical trial has revealed promising interim results for Traws Pharma, Inc.’s experimental COVID-19 treatment, ratutrelvir. This new antiviral may offer a viable alternative to the widely used Paxlovid, demonstrating fewer side effects and no reported viral rebound events among patients to date.
Interim Findings from the Clinical Trial
The findings stem from a pre-specified interim analysis of an ongoing Phase 2, randomized, open-label study that compares ratutrelvir with Paxlovid in patients diagnosed with mild-to-moderate COVID-19. The trial also includes a separate cohort of patients who are ineligible for ritonavir-boosted therapies, a demographic often limited by drug interactions or contraindications.
The analysis evaluated data from 37 patients, with 25 receiving ratutrelvir and 12 treated with Paxlovid. More than half of the planned enrollment of 90 patients has already been completed. Patients in the ratutrelvir group were administered the treatment once daily for ten days, while those in the Paxlovid group followed the standard five-day regimen.
Patient-reported outcomes indicate that symptoms were numerically comparable across both groups, based on standardized symptom diaries. However, it is essential to note that this analysis was descriptive, and no formal statistical testing was conducted.
Key Outcomes and Implications for Treatment
A significant highlight of the interim data is the absence of symptom or virologic rebound events among patients treated with ratutrelvir. In contrast, one rebound event occurred in the Paxlovid group, accounting for 8.3 percent of that cohort. This rebound phenomenon has raised concerns among healthcare providers and patients using current oral antiviral treatments.
The data further emphasized a more favorable tolerability profile for ratutrelvir. Mild dyspepsia was the most frequently reported adverse event, affecting two patients. Notably, no treatment discontinuations were documented, and common adverse effects associated with Paxlovid, such as taste disturbances and dizziness, were absent in the ratutrelvir group.
Among the six patients in the ratutrelvir arm who were ineligible for Paxlovid due to contraindications or potential drug interactions, symptom improvement patterns resembled those of the broader ratutrelvir-treated population. This finding suggests the potential utility of ratutrelvir for a demographic often excluded from antiviral therapies.
Traws Pharma executives expressed optimism regarding the absence of viral rebound, alongside the extended dosing and enhanced tolerability. They believe these factors support further evaluation of ratutrelvir not only for acute COVID-19 treatment but also for its potential role in mitigating the risk of long COVID.
Final data from this study are expected to be released in January 2026. Traws Pharma has stated that patient enrollment and follow-up are ongoing, emphasizing that larger datasets will be necessary to draw definitive conclusions regarding the efficacy and long-term outcomes of ratutrelvir.
As the COVID-19 pandemic continues to evolve, advancements in treatments like ratutrelvir offer hope for improved patient care and expanded treatment options.
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
 Health2 months ago
Health2 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-
 Science1 month ago
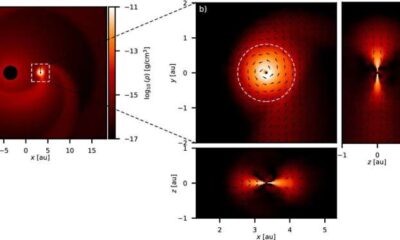
Science1 month agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-

 Lifestyle1 month ago
Lifestyle1 month agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Science2 months ago
Science2 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Lifestyle2 months ago
Lifestyle2 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle2 months ago
Lifestyle2 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Lifestyle2 months ago
Lifestyle2 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health2 months ago
Health2 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies
-

 Health2 months ago
Health2 months agoTop Hyaluronic Acid Serums for Radiant Skin in 2025
-

 Science2 months ago
Science2 months agoArizona State University Transforms Programming Education Approach
-

 Politics2 months ago
Politics2 months agoJudge Considers Dismissal of Chelsea Housing Case Citing AI Flaws








