Health
Sacituzumab Govitecan Shows Promise for HR+/HER2− Breast Cancer
Sacituzumab govitecan (SG), marketed as Trodelvy by Gilead Sciences, has demonstrated early signs of benefit for patients with hormone receptor-positive, HER2-negative (HR+/HER2−) metastatic breast cancer, according to findings from the phase 3 ASCENT-07 trial (NCT05840211). These results were presented at the 2025 San Antonio Breast Cancer Symposium, highlighting SG’s potential impact on overall survival.
Study Design and Methodology
The ASCENT-07 trial involved 690 adults with HR+/HER2− locally advanced unresectable or metastatic breast cancer. Participants were randomly assigned to receive SG at a dose of 10 mg/kg intravenously or a chemotherapy regimen of the physician’s choice, which included capecitabine, nab-paclitaxel, or paclitaxel. Randomization was stratified based on prior use of CDK4/6 inhibitors, HER2 immunohistochemistry status, and geographic region.
The primary endpoint was progression-free survival (PFS), assessed by blinded independent central review (BICR) according to RECIST v1.1 criteria. Key secondary endpoints included overall survival (OS), objective response rate (ORR), and quality of life, along with other safety and duration of response assessments.
Primary Results from ASCENT-07
Of the 690 participants, 456 received SG and 234 received the chemotherapy regimen. The median age of participants was approximately 57 years, with a significant proportion having previously received CDK4/6 inhibitors (SG: 93%; TPC: 92%).
At a median follow-up of 15.4 months, SG did not show a statistically significant improvement in PFS compared to the chemotherapy regimen (hazard ratio [HR] of 0.85; 95% confidence interval [CI], 0.69–1.05; P = 0.130). However, investigator assessments indicated a numerical improvement in PFS for SG (HR of 0.78; 95% CI, 0.64–0.93; nominal P = 0.008).
While OS data were still immature at the primary analysis (27% maturity), an early trend favored SG with a HR of 0.72 (95% CI, 0.54–0.97; nominal P = 0.029). The ORR by BICR was similar for both groups (SG: 37%; TPC: 33%), while the median duration of response was longer with SG (12.1 months versus 9.3 months for TPC).
The most common grade 3 or higher treatment-emergent adverse event was neutropenia, affecting 56% of SG recipients compared to 21% for the chemotherapy group. Despite the higher incidence of severe adverse events with SG, the rates of dose reductions were comparable between the two groups, and fewer participants discontinued treatment with SG compared to TPC.
In conclusion, while the ASCENT-07 trial did not meet its primary endpoint of PFS as assessed by BICR, it revealed promising trends towards improved OS with SG. No new safety concerns were identified during the trial. These findings indicate that although SG may not significantly extend PFS for patients in this setting, its tolerability and potential OS benefits warrant further investigation.
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
 Health2 months ago
Health2 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-
 Science1 month ago
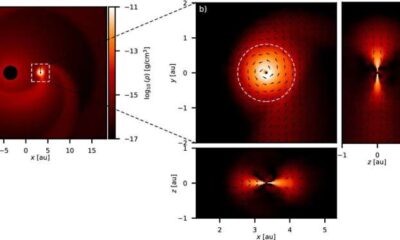
Science1 month agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-

 Lifestyle1 month ago
Lifestyle1 month agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Science2 months ago
Science2 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Lifestyle2 months ago
Lifestyle2 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle2 months ago
Lifestyle2 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Lifestyle2 months ago
Lifestyle2 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health2 months ago
Health2 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies
-

 Health2 months ago
Health2 months agoTop Hyaluronic Acid Serums for Radiant Skin in 2025
-

 Science2 months ago
Science2 months agoArizona State University Transforms Programming Education Approach
-

 Politics2 months ago
Politics2 months agoJudge Considers Dismissal of Chelsea Housing Case Citing AI Flaws








