Health
Nektar Therapeutics Faces Crucial Test Amid Share Decline

Nektar Therapeutics is poised for a critical evaluation as it prepares to release results from its Phase 2 study on a treatment for moderate-to-severe eczema, known medically as atopic dermatitis. Following a significant decline in share value—over 40% since November—investors are closely watching the upcoming data expected this month.
The results will be pivotal in determining the company’s risk-reward profile moving forward. The maintenance efficacy benchmark is currently set by established treatments such as Dupixent and Ebglyss, both of which demonstrate a sustained response in approximately 70% of atopic dermatitis patients. These patients achieve an EASI75 response, meaning they experience a significant reduction in skin lesions, after a 16-week induction period.
Market Dynamics and Competitive Landscape
The landscape for atopic dermatitis treatments is becoming increasingly competitive. As Nektar Therapeutics navigates this challenging environment, the one-year maintenance results from its rezpeg study will play a crucial role in its market positioning. If the company can match or exceed the efficacy of Dupixent and Ebglyss, it could rekindle investor confidence and potentially reverse the recent downturn in stock performance.
Investors will be particularly attentive to how Nektar’s treatment compares quantitatively with these existing therapies. The EASI75 response rate has become a standard measurement for success in this sector, making the stakes particularly high for Nektar as it seeks to establish its treatment’s viability and commercial potential.
Implications for Patients and Future Developments
The results from this trial could have significant implications not only for Nektar but also for the thousands of patients suffering from atopic dermatitis. A successful outcome could lead to new treatment options for a condition that affects millions globally, providing hope for improved quality of life.
In the coming weeks, Nektar Therapeutics will unveil the data from its Phase 2 study, which could mark a turning point for the company. As the healthcare industry watches closely, the focus remains on the efficacy of rezpeg and how it can compete against established treatments. The results will not only affect market perceptions but also the future trajectory of Nektar Therapeutics in the competitive biopharmaceutical landscape.
-

 Science3 months ago
Science3 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
 Science3 months ago
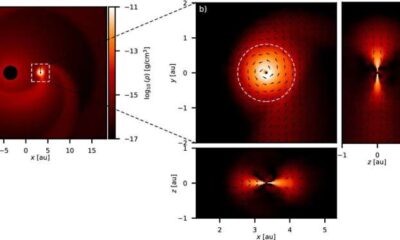
Science3 months agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-
 Health3 months ago
Health3 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-

 Health2 months ago
Health2 months ago$2.2 Million Boost for Cancer Research and Training in Hawaiʻi
-

 Lifestyle3 months ago
Lifestyle3 months agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Health1 month ago
Health1 month agoSacituzumab Govitecan Shows Promise for HR+/HER2− Breast Cancer
-

 Lifestyle3 months ago
Lifestyle3 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Science4 months ago
Science4 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Science3 months ago
Science3 months agoInterstellar Comet 3I/ATLAS Approaches Sun, No Threat to Earth
-

 Lifestyle4 months ago
Lifestyle4 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle4 months ago
Lifestyle4 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health4 months ago
Health4 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies