Health
Intranasal Vaccine Shows Promise Against H5N1 in Early Trial

Researchers at the University of Maryland School of Medicine have reported promising results from an early-phase clinical trial investigating an intranasal vaccine designed to combat multiple strains of H5N1, commonly known as “bird flu.” The study, published in the journal Nature Communications, underscores the potential of mucosal immunization strategies, where vaccines are delivered through the nostrils, to enhance immune responses against various influenza strains.
The trial’s corresponding author, Justin Ortiz, MD, MS, a Professor of Medicine at UMSOM, emphasized the importance of developing effective countermeasures against H5N1. He stated, “The spread of H5N1 influenza in animals with spillover into human populations globally highlights the critical need for effective countermeasures to protect our communities from this and other pathogens with pandemic potential.” Ortiz believes this intranasal, shelf-stable vaccine could significantly contribute to pandemic preparedness.
Current influenza vaccines, typically administered intramuscularly, primarily elicit systemic immune responses. While effective in preventing symptomatic illness when well-matched to circulating strains, these vaccines may not adequately prevent transmission. In contrast, the intranasal vaccine aims to stimulate immune defenses at the site of infection, offering a novel approach to reduce viral spread.
Clinical Trial Insights
The randomized, controlled trial involved 40 healthy adult volunteers who were assigned to receive different doses of the H5 flu vaccine combined with BlueWillow’s NanoVax W 80 5EC adjuvant. Control groups were administered either a placebo or a high dose of the H5 vaccine without the adjuvant. Six months later, all participants received an intramuscular booster of the H5 flu vaccine.
The findings indicated that the NanoVax H5 intranasal vaccine was both safe and well tolerated among participants. Notably, only those who received the boosted nasal vaccine exhibited strong immune “priming,” preparing their immune systems for a robust response when later given an intramuscular H5 flu shot. Even without a booster, the NanoVax H5 vaccine effectively triggered mucosal and systemic immune responses, a significant advancement compared to other intranasal recombinant H5 flu vaccines that have not achieved similar results in clinical trials.
Study co-lead author Meagan E. Deming, MD, Ph.D., noted the vaccine’s capacity to help the immune system recognize various versions of the H5N1 virus, which is crucial given the virus’s propensity to mutate. She stated, “The use of the adjuvant also suggests this approach might allow for lower doses of the vaccine, which could make our current vaccine stocks available to more people in the event of an outbreak.”
Implications for Pandemic Preparedness
The research revealed that volunteers receiving the adjuvanted H5 vaccine demonstrated heightened immune activity, including increased levels of protective antibodies and improved ability to destroy infected cells. Franklin R. Toapanta, MD, Ph.D., another co-lead author, remarked, “These findings demonstrate successful mucosal priming and the potential for broad cross-clade immunity.” He highlighted the intranasal vaccine’s ability to elicit both mucosal and cellular immune responses as a significant advancement in influenza prevention strategies.
Mark T. Gladwin, MD, Dean of the University of Maryland School of Medicine, reiterated the study’s alignment with global public health priorities. He stressed the need for vaccines that reduce transmission and provide broader protection against emerging influenza strains. He also called attention to the necessity for further exploration of mucosal immune biomarkers and alternative immune correlates of protection to expedite the development of intranasal influenza vaccines.
The study, titled “An Intranasal Adjuvanted, Recombinant Influenza A/H5 Vaccine Primes Against Diverse H5N1 Clades: A Phase I Trial,” highlights a significant step forward in the ongoing fight against influenza viruses with pandemic potential. As global health initiatives continue to evolve, the implications of this research could pave the way for more effective influenza vaccination strategies in the future.
-
 Health2 months ago
Health2 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
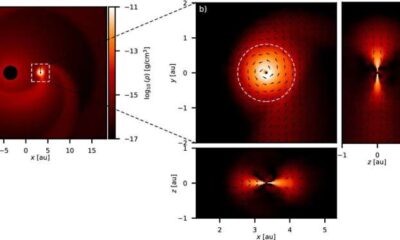
 Science1 month ago
Science1 month agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-

 Lifestyle1 month ago
Lifestyle1 month agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Science2 months ago
Science2 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Lifestyle2 months ago
Lifestyle2 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle2 months ago
Lifestyle2 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Lifestyle2 months ago
Lifestyle2 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health2 months ago
Health2 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies
-

 Health2 months ago
Health2 months agoTop Hyaluronic Acid Serums for Radiant Skin in 2025
-

 Politics2 months ago
Politics2 months agoJudge Considers Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Science2 months ago
Science2 months agoArizona State University Transforms Programming Education Approach