Politics
Trump’s Order to Reschedule Marijuana Promises Changes Ahead

President Donald Trump’s recent executive order to reschedule marijuana has the potential to reshape the cannabis landscape in the United States, bringing immediate changes for businesses, but experts warn that the path forward is complex. The order, issued on December 18, 2023, aims to lower the classification of cannabis under the Controlled Substances Act from Schedule I to Schedule III. This shift is designed to facilitate increased research and alleviate some regulatory burdens on cannabis companies.
Despite the optimism surrounding this move, Gillian Schauer, executive director of the nonpartisan Cannabis Regulators Association, cautioned that the reality is not as straightforward as it may seem. “It’s hard to see the big headlines of, ‘Marijuana rescheduled to Schedule III; marijuana research will open,’” she noted, emphasizing that the executive order alone cannot change over 50 years of entrenched federal drug policy.
The authority to reschedule a drug does not rest solely with the presidency. Schauer explained that the Controlled Substances Act does not allow unilateral action by the President. Instead, changes typically require a formal rulemaking process or new legislation from Congress. How the current administration proceeds will significantly impact both the timeline and the extent of any reforms.
Potential Pathways for Rescheduling
The future of marijuana rescheduling depends on the approach taken by the Department of Justice (DOJ). Trump’s order directs Pam Bondi, the Attorney General, to take “all necessary steps to complete the rulemaking process related to rescheduling marijuana to Schedule III” as quickly as possible. This directive could either continue the process initiated under former President Joe Biden or pursue a more expedited route.
Historically, the DOJ has utilized a provision in the Controlled Substances Act that allows for the rescheduling of drugs without the lengthy procedures normally required. This method was previously applied in 2018 for the CBD epilepsy drug Epidiolex, which was classified in Schedule V. Schauer indicated that this expedited process might be employed again, potentially avoiding a lengthy public comment period.
Nevertheless, if the DOJ opts for the traditional notice-and-comment method, the timeline for rescheduling could extend significantly. Given the intense public interest—over 43,000 comments were submitted for the DEA’s earlier proposed rescheduling rule—any public comment period would likely be filled with contributions from a wide range of stakeholders.
Impacts on Cannabis Businesses
For cannabis companies, immediate benefits could include relief from the notorious 280E tax code, which has long hindered profitability. Under current regulations, cannabis businesses cannot deduct common expenses due to their classification as Schedule I drugs, leading to an inflated effective tax rate. Sam Brill, CEO of Ascend Wellness Holdings, expressed optimism that rescheduling could alleviate this burden, stating, “The biggest thing that happens overnight is the 280E…would no longer apply.”
Beyond tax relief, Brill also highlighted the hope that rescheduling might eventually lead to improved banking options for the cannabis industry. Currently, many financial institutions refrain from providing services to legal cannabis operations due to potential liabilities. “The lack of the use of a credit card is really one of the biggest challenges for customers,” he explained, noting the cash-based nature of many transactions in the industry.
Challenges in Medical Research
While the rescheduling of marijuana is expected to enhance avenues for medical research, significant obstacles remain. Scientists have previously highlighted the arduous process of obtaining Schedule I licenses, which the new rules would alleviate. Staci Gruber, a neuroscientist at McLean Hospital and Harvard Medical School, pointed out that while the changes would ease some regulatory burdens, the challenge of sourcing marijuana for research persists.
Researchers are currently required to obtain cannabis from a limited number of sources, which has improved from previous decades when they were restricted to a single facility at the University of Mississippi. Schauer noted that even with rescheduling, “there’s a lot that will still be challenging in researching cannabis unless we see a lot of agency policies change and adjust.”
As the administration moves forward with the executive order, the implications for both the cannabis industry and research community will unfold. Stakeholders across the spectrum are keenly watching how this significant policy shift will impact the future of marijuana in the United States, while the balance between expediency and legal scrutiny will shape the administration’s approach.
-
 Health2 months ago
Health2 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
 Science1 month ago
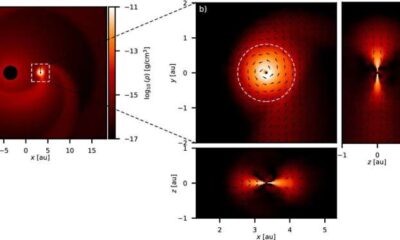
Science1 month agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-

 Lifestyle1 month ago
Lifestyle1 month agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Science2 months ago
Science2 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Lifestyle2 months ago
Lifestyle2 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle2 months ago
Lifestyle2 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Lifestyle2 months ago
Lifestyle2 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health2 months ago
Health2 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies
-

 Health2 months ago
Health2 months agoTop Hyaluronic Acid Serums for Radiant Skin in 2025
-

 Science2 months ago
Science2 months agoArizona State University Transforms Programming Education Approach
-

 Politics2 months ago
Politics2 months agoJudge Considers Dismissal of Chelsea Housing Case Citing AI Flaws









