Health
Dangerous Baby Formula Lingers on Store Shelves Despite Recall

Concerns surrounding food safety have intensified as dangerous products continue to remain available on store shelves even after official recalls. A recent incident involving ByHeart powdered infant formula has drawn significant attention, highlighting the challenges retailers face in removing recalled items from sale.
During a routine grocery trip just before Christmas, a shopper at a Kroger store spotted a can of ByHeart powdered infant formula with a recall notice dated November 11, 2023, taped underneath. The shopper quickly documented the find, sending a photo to experts in food safety. The reaction was unanimous: this situation is “nuts,” according to food safety attorney Bill Marler, who represents families affected by botulism linked to the formula.
On the same day, Marler amended legal complaints to include retailers, asserting that they failed to act swiftly enough to remove the dangerous product from their shelves. In response to queries regarding the incident, Kroger’s press office stated, “When the recall was issued, we urgently removed the affected product and immediately placed a block at the point of sale to make it impossible for a customer to purchase the recalled item.” However, the company did not clarify how the formula was left on the shelf despite these measures.
The U.S. Food and Drug Administration (FDA) issued warning letters on December 12 to Kroger and three other retailers—Target, Walmart, and Albertsons—after inspectors discovered cans of ByHeart formula for sale across 36 states. The FDA’s actions highlighted a recurring issue: companies often struggle to effectively remove recalled products from their inventory.
In 2022, the Consumer Product Safety Commission (CPSC) penalized TJX, the parent company of TJ Maxx and HomeGoods, with a civil penalty of $13 million for selling over 1,200 recalled items, including infant sleepers known to be dangerous. A TJX spokesperson expressed regret, stating they have invested significantly in improving their processes to prevent such occurrences.
The situation raises questions about compliance and communication within retailers. Peter Feldman, the acting chairman of the CPSC, remarked on the commonality of such issues, emphasizing that once a product is recalled, it becomes illegal to sell. Stores may encounter obstacles in disseminating recall information to all employees, leading to lapses in compliance. Some retailers have even been accused of disabling inventory control systems during peak shopping times, inadvertently allowing the sale of recalled goods.
Both the CPSC and the FDA conduct checks to ensure recalled products are not available for sale, although these checks are not exhaustive. The CPSC’s dedicated ESAFE team actively monitors online marketplaces and has issued over 33,000 takedown orders in the past three months, a significant increase from the previous year. They focus on categories like baby products and electronics, which are particularly vulnerable to resale after recalls.
The increase in recalls is alarming. In fiscal year 2023, the CPSC reported 357 recalls, up from 238 in 2020. The growing number of recalls has raised concerns about whether regulatory bodies have sufficient resources to monitor compliance effectively.
The ByHeart formula recall stemmed from an investigation by the California Department of Public Health and the CDC, which identified a rise in infant botulism cases linked to the product. Testing confirmed the presence of botulinum spores in several cans. This serious condition can severely impact infants, leading to hospitalization and requiring costly treatments like BabyBIG, a therapeutic product that helps counteract the toxin.
As of December 17, the FDA reported that 51 infants across 19 states had been affected by the botulism cases. Fortunately, no fatalities have been reported, thanks in part to timely medical interventions.
Former FDA deputy commissioner Frank Yiannis criticized the slow response to the ByHeart recall, noting that the initial notification only included two lots of the formula, leading to confusion. He emphasized the need for better communication and prompt action from both companies and regulatory agencies. Yiannis also advocated for the adoption of advanced technology, such as RFID tags, to enhance product tracking and improve recall efficiency.
In a statement regarding the recall, ByHeart expressed regret over the distress caused to customers and partners, noting that they have paused production while auditing their supply chain for potential contamination sources. The company urges parents to monitor for symptoms of infant botulism, reiterating that recalls should serve as a critical safety measure for consumers.
As issues with recalled products persist, advocacy groups emphasize the need for stricter adherence to safety protocols. Sandra Eskin, CEO of the nonprofit group Stop Foodborne Illness, stressed the importance of accountability among large companies in ensuring consumer safety. The current situation underscores the ongoing challenges in the recall process and the need for vigilance among consumers when purchasing food products.
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
 Health2 months ago
Health2 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-
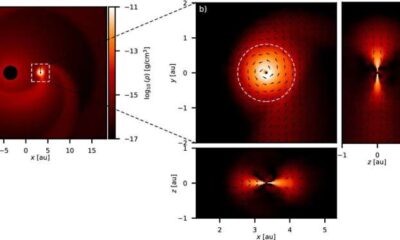
 Science2 months ago
Science2 months agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-

 Lifestyle1 month ago
Lifestyle1 month agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Science3 months ago
Science3 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Lifestyle3 months ago
Lifestyle3 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle2 months ago
Lifestyle2 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Lifestyle3 months ago
Lifestyle3 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health3 months ago
Health3 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies
-

 Health3 months ago
Health3 months agoTop Hyaluronic Acid Serums for Radiant Skin in 2025
-

 Science3 months ago
Science3 months agoArizona State University Transforms Programming Education Approach
-

 Politics2 months ago
Politics2 months agoJudge Considers Dismissal of Chelsea Housing Case Citing AI Flaws