Business
FDA Selects Nine Products for Accelerated Review Program

The U.S. Food and Drug Administration (FDA) has announced the selection of nine therapies for its new pilot program designed to expedite the regulatory review process for products deemed to meet national priority goals. This initiative aims to accelerate the approval timeline from the standard review period of 10 to 12 months to just one to two months for selected therapies.
Among the first recipients of the Commissioner’s National Priority Review Voucher (CNPV) are a gene therapy for hearing loss, a smoking cessation drug, and a domestically manufactured anesthetic. The program, introduced in June 2023, is intended to address significant public health needs and enhance national security through local production of essential medications.
Details on the CNPV Program
Each of the nine selected products has been nominated by one of the FDA’s 27 review divisions, which are organized according to therapeutic areas. The FDA’s Office of New Drugs oversees this initiative, with each division responsible for identifying products that align with the CNPV program’s objectives. In addition to nominations, pharmaceutical companies can submit applications for consideration by the relevant review division.
The FDA plans to implement a collaborative, team-based approach akin to a tumor board meeting in oncology. This method brings together specialists from various fields to discuss and evaluate the best treatment options for patients. Following the completion of the review processes for the products awarded a CNPV, the FDA will conduct one-day meetings to deliberate on the applications.
Despite the expedited review period, the FDA has made it clear that safety remains its top priority. As stated by Martin Makary, FDA Commissioner, “We like speed, but we don’t like cutting any corners on safety.” The FDA retains the right to extend review times if applications are incomplete or if manufacturing concerns arise.
Selected Products and Their Potential Impact
The nine therapies selected for this program include:
1. **Regeneron Pharmaceuticals’ DB-OTO**: A gene therapy aimed at treating a rare genetic form of hearing loss.
2. **Revolution Medicines’ RMC-6236**: Developed for pancreatic cancer, this drug aims to address a significant medical need.
3. **Disc Medicine’s bitopertin**: This therapy could potentially be the first disease-modifying treatment for the rare blood disorder known as erythropoietic protoporphyria.
4. **Dompé’s cenegermin (brand name Oxervate)**: While the eye drop formulation is already approved for treating neurotrophic keratitis, Dompé seeks to gain expedited approval for an intranasal version intended for non-arteritic anterior ischemic optic neuropathy.
5. **Phlow’s ketamine anesthetic**: This product is noteworthy as it will be manufactured domestically, addressing a national security concern due to a lack of local suppliers for the active pharmaceutical ingredient.
6. **Augmentin XR**: This established antibiotic has also received a voucher for domestic manufacturing.
7. **EMD Serono’s pergoveris**: A medication aimed at treating infertility.
8. **Sanofi’s teplizumab (brand name Tzhield)**: Approved for delaying advanced type 1 diabetes, this drug is another critical addition to the list.
9. **Achieve Life Sciences’ cytisinicline**: This drug is in development as a smoking and vaping cessation aid.
By selecting these products, the FDA aims to not only address pressing health issues but also to stimulate domestic manufacturing and reduce drug prices to enhance accessibility for patients.
As the FDA moves forward with this initiative, it anticipates announcing more recipients of the CNPV in the coming months, further expanding its efforts to prioritize public health and safety.
-
 Health2 months ago
Health2 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
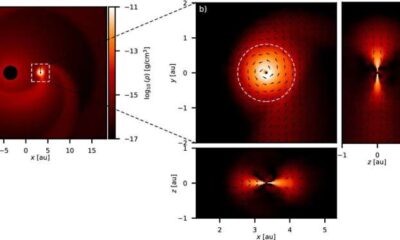
 Science1 month ago
Science1 month agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-

 Lifestyle1 month ago
Lifestyle1 month agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Science2 months ago
Science2 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Lifestyle2 months ago
Lifestyle2 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle2 months ago
Lifestyle2 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Lifestyle2 months ago
Lifestyle2 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health2 months ago
Health2 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies
-

 Health2 months ago
Health2 months agoTop Hyaluronic Acid Serums for Radiant Skin in 2025
-

 Science2 months ago
Science2 months agoArizona State University Transforms Programming Education Approach
-

 Politics2 months ago
Politics2 months agoJudge Considers Dismissal of Chelsea Housing Case Citing AI Flaws








