Science
AI Model Predicts Cell Morphology, Reducing Experimental Costs

A team of researchers from MBZUAI has developed an innovative artificial intelligence model named MorphDiff that predicts how cells will change in response to drugs or gene edits without the need for extensive trial and error. Their findings, published in Nature Communications, could significantly streamline the drug discovery process and reduce costs associated with experimental research.
Predicting cellular changes traditionally involves a combination of scientific knowledge, artistic insight, and costly experimentation. The conventional approach requires extensive imaging and analysis, making it impractical to explore millions of conditions. MorphDiff addresses this challenge by simulating “after” images of cells based on molecular readouts, allowing researchers to visualize changes before conducting experiments.
At its core, MorphDiff employs a diffusion model that utilizes the transcriptome, which reflects the pattern of gene expression altered by various perturbations. This approach transforms the existing workflow by leveraging high-throughput imaging, a recognized method for uncovering the mechanisms behind compounds and assessing their bioactivity. By learning from instances where both gene expression and cell morphology data are available, MorphDiff generates realistic images based solely on the L1000 gene expression profile.
The model integrates two key components: a Morphology Variational Autoencoder (MVAE) that compresses five-channel microscope images and reconstructs them with high fidelity, and a Latent Diffusion Model that denoises samples within a compact latent space. This dual approach allows MorphDiff to produce both gene-to-image (G2I) generation and image-to-image (I2I) transformation, facilitating a seamless transition between control images and their perturbed counterparts.
The researchers demonstrated that MorphDiff achieves competitive fidelity when compared to traditional image generation techniques. In tests across various datasets, including genetic and drug-related splits, MorphDiff consistently outperformed standard baselines in terms of fidelity and diversity. Notably, the model successfully captured biological features, aligning closely with real data, and achieving over 70 percent indistinguishability in generated feature distributions compared to ground truth.
In practical terms, MorphDiff’s capabilities extend to thousands of treatments, with the potential to enhance mechanism-of-action (MOA) retrieval. When researchers query a profile, MorphDiff helps identify reference drugs with similar mechanisms, surpassing previous image-generation models and approaching the accuracy of actual images. The average improvement over existing methods in top-k retrieval experiments was 16.9 percent compared to the strongest baseline.
Despite its advancements, the study acknowledges certain limitations. The inference process remains relatively slow, and the model does not explicitly encode factors such as time and concentration due to data constraints. Future enhancements may involve incorporating newer sampling methods and additional conditions informed by matched datasets.
In essence, MorphDiff serves as a valuable tool for researchers, particularly those with access to the extensive L1000 library but limited imaging resources. By generating predicted morphologies for new compounds, the model can cluster them based on similarity to known mechanisms, guiding researchers on which candidates to prioritize for imaging and validation.
This breakthrough emphasizes the growing role of generative AI in scientific research, particularly in capturing complex cellular phenotypes and preserving critical relationships. While MorphDiff will not replace traditional microscopy, it promises to reduce the number of experiments necessary to identify significant findings, ultimately leading to more efficient research practices and cost savings within the scientific community.
-
 Health2 months ago
Health2 months agoNew Gel Offers Hope for Regrowing Tooth Enamel in Dentistry
-

 Science2 months ago
Science2 months agoUniversity of Hawaiʻi at Mānoa Joins $25.6M AI Initiative for Disaster Monitoring
-
 Science1 month ago
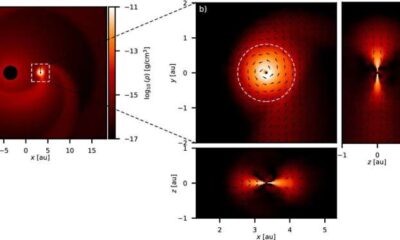
Science1 month agoALMA Discovers Companion Orbiting Red Giant Star π 1 Gruis
-

 Lifestyle1 month ago
Lifestyle1 month agoPark Jung Min’s Endearing Moment with Hwasa Steals Show at Awards
-

 Science2 months ago
Science2 months agoIROS 2025 to Showcase Cutting-Edge Robotics Innovations in China
-

 Lifestyle2 months ago
Lifestyle2 months agoStone Island’s Logo Worn by Extremists Sparks Brand Dilemma
-

 Lifestyle2 months ago
Lifestyle2 months agoSampson County Celebrates Susie Faison’s 100th Birthday Milestone
-

 Lifestyle2 months ago
Lifestyle2 months agoMary Morgan Jackson Crowned Little Miss National Peanut Festival 2025
-

 Health2 months ago
Health2 months agoStartup Liberate Bio Secures $31 Million for Next-Gen Therapies
-

 Health2 months ago
Health2 months agoTop Hyaluronic Acid Serums for Radiant Skin in 2025
-

 Politics2 months ago
Politics2 months agoJudge Considers Dismissal of Chelsea Housing Case Citing AI Flaws
-

 Science2 months ago
Science2 months agoArizona State University Transforms Programming Education Approach









